Canabrava Ring Surgical procedure
Complete the following questionnaire



Worldwide Canabrava Ring Launch
MORE INFORMATION

SPECS
| Brand name | CANABRAVA RING |
| Reference | CANA-RING |
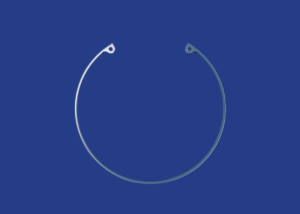
| Description and indications | An iris expander ring desinged to safely expand the iris in order to simplify phacoemulsification, as well as surgeries in which the pupil does not dilate properly using pharmaceuticals. It provides a pupillary opening of 6.3 mm and keeps dilation stable during surgery, improving the surgeon´s access and field of vision. • 300° semicircular ring with a 60° opening made from a single piece. • Does not require an injector. • Easy to adapt and remove. • The ring can be inserted in incisions smaller than 2 mm. • Easy to insert in pupils smaller than 4.5 mm and in shallow chambers. • Optimum visibility. • Disposable device that cannot be re-sterilized. Indications: • Myosis. • Coloboma. • Traumatic sector iridectomy. • Intraoperative floppy iris syndrome. • Iris prolapse. • Small pupils. • Rigid iris tissue due to a pseudoexfoliation. • Vitrectomy with small pupils. |
| Material | Medical grade Polymethyl Methacrylate (PMMA). |
| Sterilization method | Ethylene Oxide (ETO). |
| Packaging | Sterile, single unit. |
| Shelf-life | 4.5 years |
CE 0318