
ESPECIFICATIONS
| Brand name | AJL OOKP |
| Reference | AJL-OOKP |
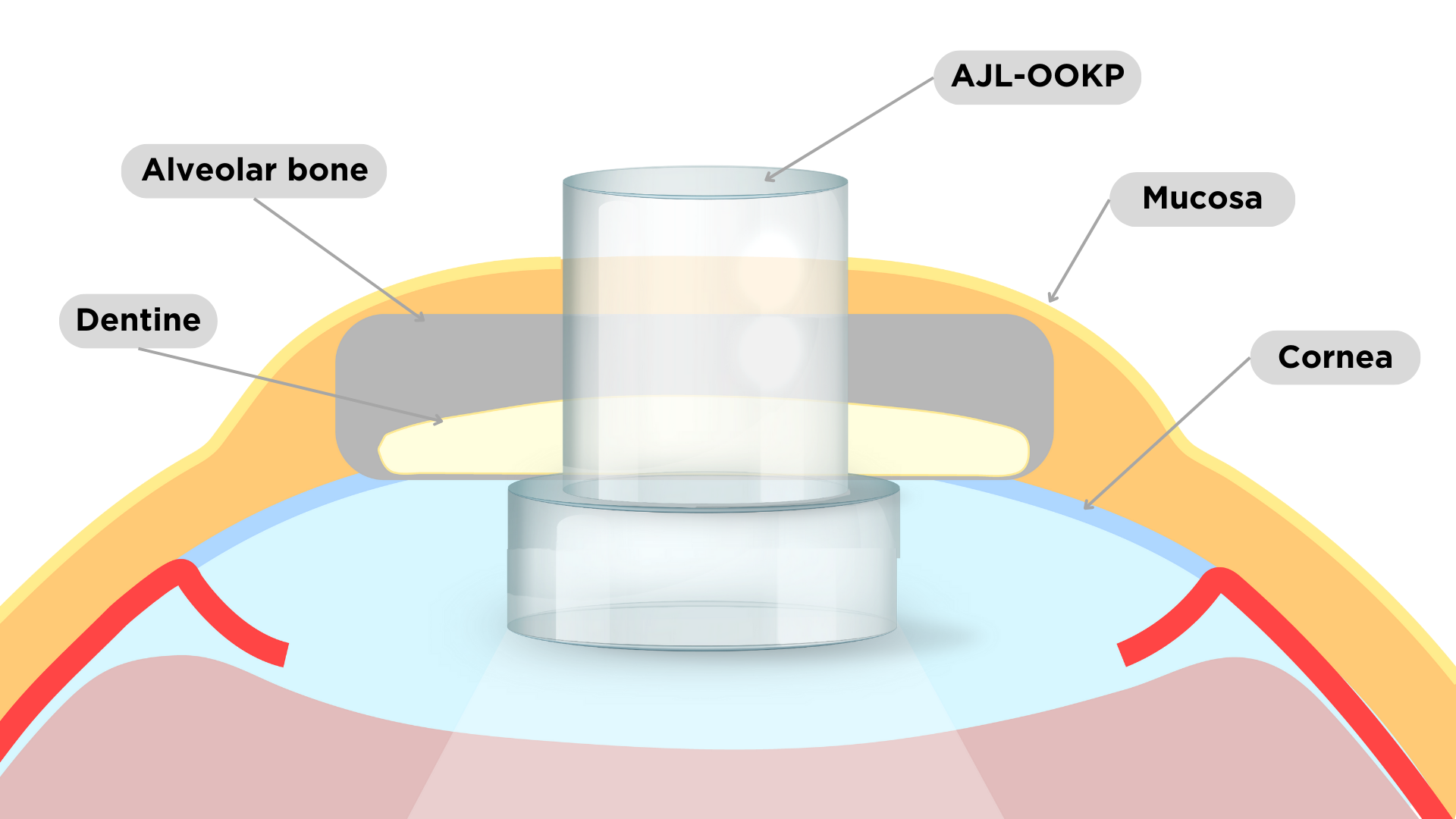
| Product description and indications | The osteo-odonto-keratoprosthesis is a medical device implantable in the patient’s cornea with a transparent cylinder shape supported by an autologus tissue (canine tooth or tibia bone). It enables to restore sight to an eye in cases of corneal opacity that prevents vision and a new corneal transplant is not possible: - Autoimmune diseases such as Stevens-Johnson syndrome and ocular pemphigoid - Severe dry eye - Severe chemical burns affecting both eyes |
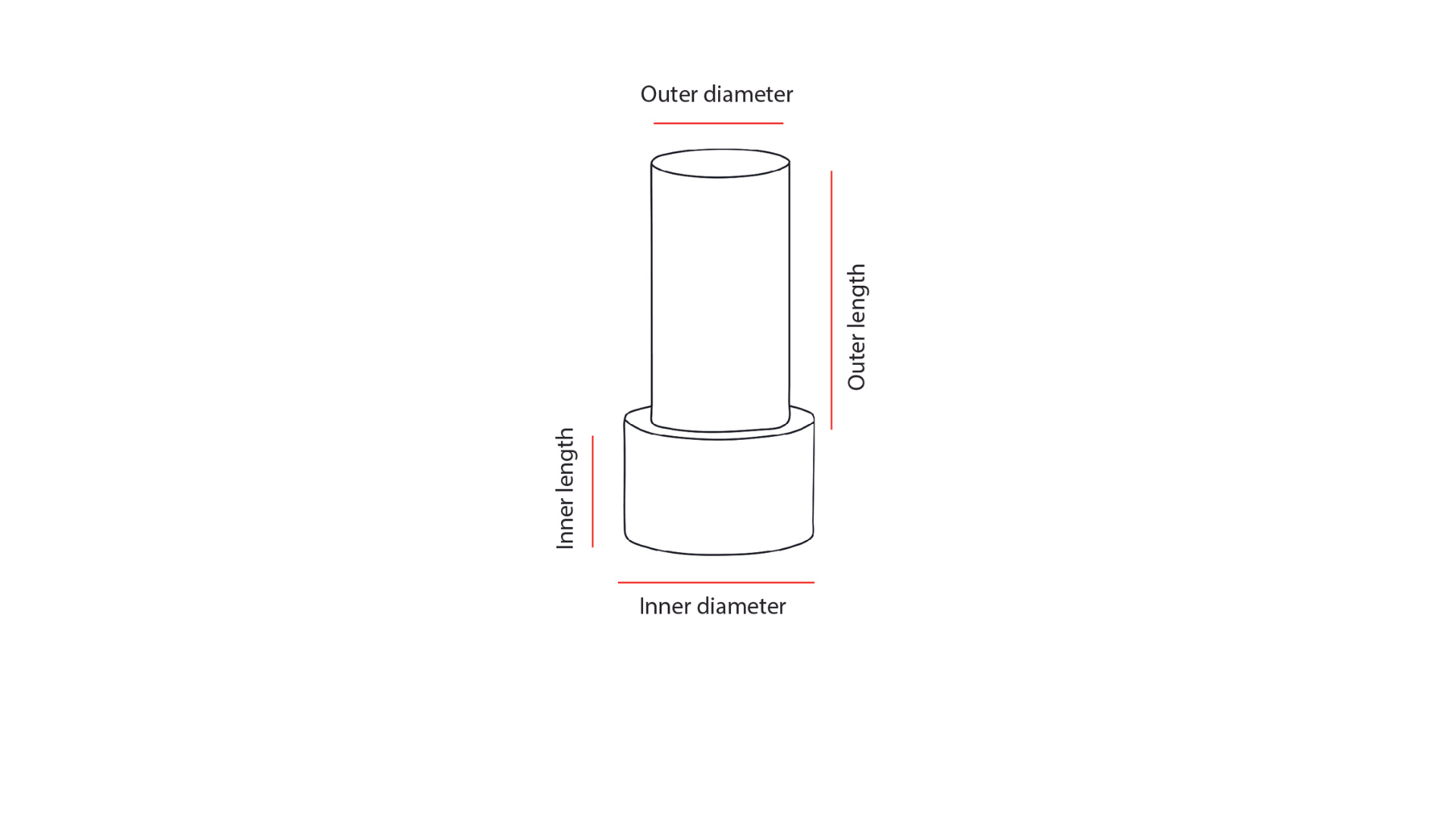
| Implant specifications | Custom made device |
| Ordering documentation | - Completed custom made device order specifying the axial length of the eye and additional data. - OCT of the anterior segment |
| Material | Polymethyl Methacrylate (PMMA) |
| Sterilization method | Ethylene Oxide (ETO) |
| Supply | Sterile packed single implant |
| Shelf-life | 4.5 years |
MORE INFORMATION
tainer]














