AJL OOKP
Osteo-odonto-keratoprosthesis

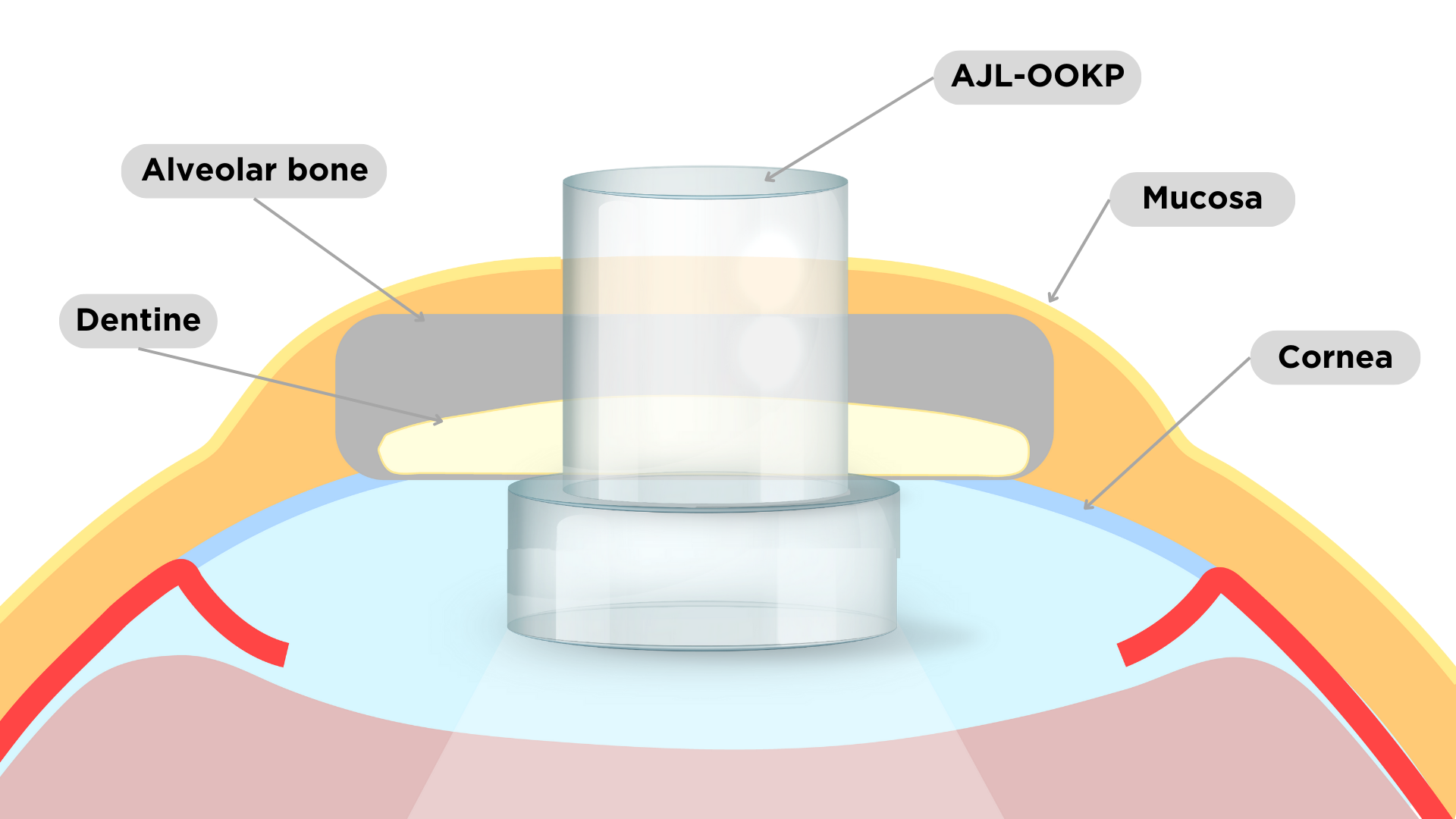
The osteo-odonto-keratoprosthesis is a medical device implantable in the cornea with a transparent cylinder shape supported by an autologus tissue (canine tooth or tibia bone).
It enables to restore sight to an eye in cases of corneal opacity that avoids vision and a new corneal transplant is not possible:

ESPECIFICATIONS
| Brand name | AJL OOKP |
| Reference | AJL-OOKP |
| Product description and indications | The osteo-odonto-keratoprosthesis is a medical device implantable in the cornea with a transparent cylinder shape supported by an autologus tissue (canine tooth or tibia bone). It enables to restore sight to an eye in cases of corneal opacity that avoids vision and a new corneal transplant is not possible: - Autoimmune diseases such as Stevens-Johnson syndrome and ocular pemphigoid - Severe dry eye - Severe chemical burns a ecting both eyes |
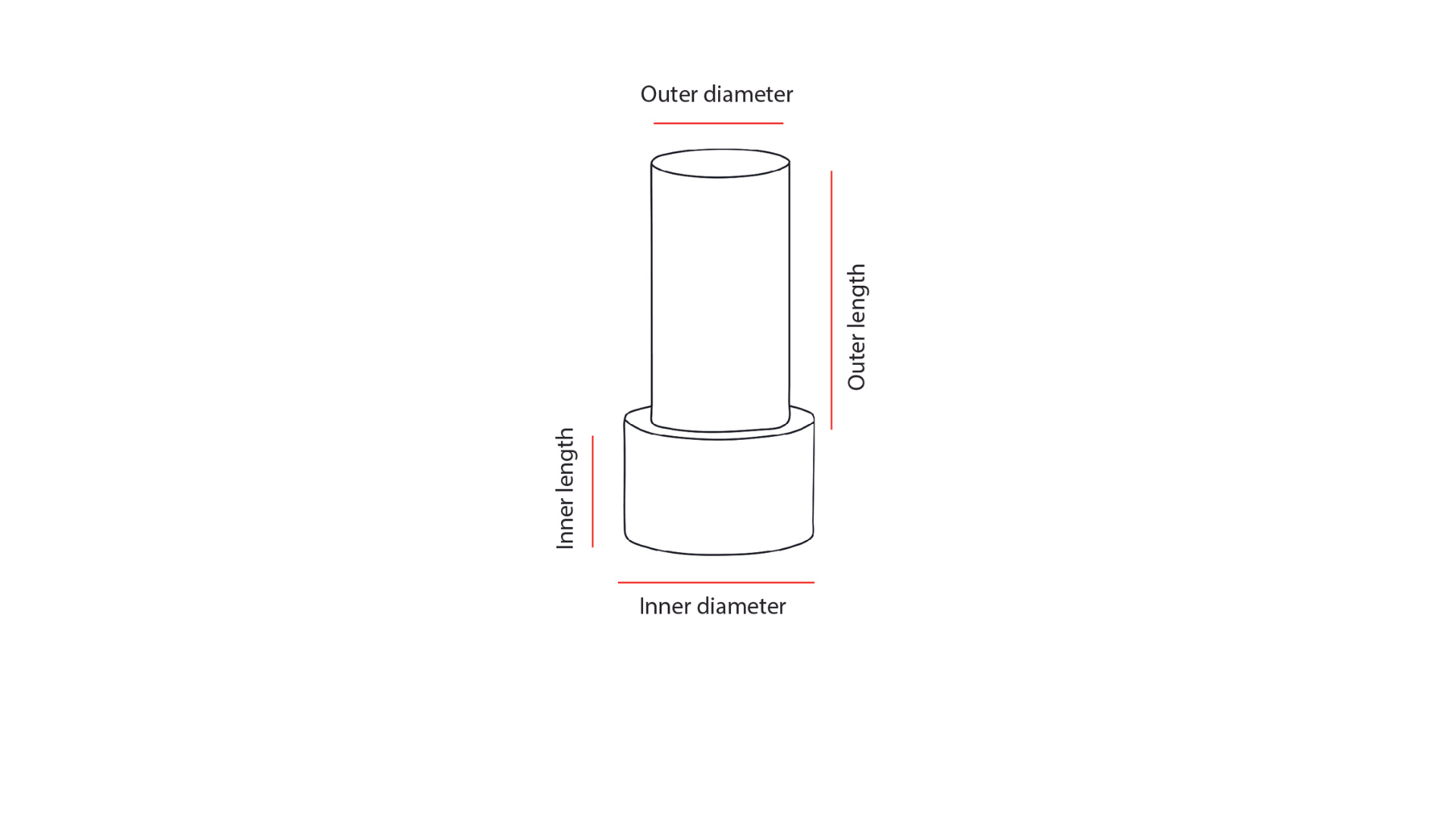
| Implant specifications | Custom made device |
| Ordering documentation | - Complete the order form specifying your patient's axial length and additional device data. - Anterior segment OCT |
| Material | Polymethyl Methacrylate (PMMA) |
| Sterilization method | Ethylene Oxide (ETO) |
| Supply | Single use packaging |
| Shelf-life | 4.5 years |
MORE INFORMATION